GlucoModicum Completes Clinical Performance Study of 646 Subject Visits and Reports Positive Results for Its Sofio Needle-Free Glucose Monitor
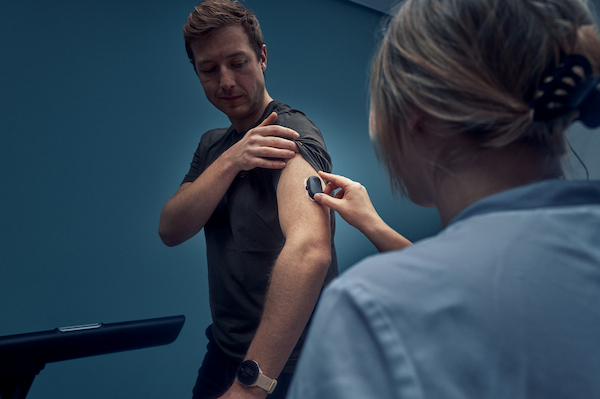
GlucoModicum has completed a comprehensive clinical performance study involving 646 patient visits, in which both individuals with type 2 diabetes and healthy volunteers used the Sofio needle-free glucose monitor.
The study included multi-hour standardized glucose tolerance tests as well as ambulatory use, where participants wore Sofio during daily activities and exercise.
Different versions of Sofio system were tested and all tested sensors were based on Sofio’s mass manufacturing blueprint. The best performing system demonstrated a mean absolute relative difference (MARD) of 11.5%.
The study covered a broad and diverse participant profile to ensure performance across varied physiological and demographic backgrounds.
The study participant profile and range were as follows:
- Visit count: over 600 participant visits, yielding >1,500 device-wear sessions
- Health condition: individuals with type 2 diabetes, prediabetes and healthy volunteers
- Gender distribution: 51% female, 49% male
- Age range: 21 to 75 years
- Nationalities: primarily Finnish participants, while Sofio (GlucoModicum) own team members have tested and worn Sofio actively, representing ~20 different nationalities.
- Blood glucose range during testing: 2.6 mmol to 26.4 mmol
- HbA1c range: 4.7% to 9.9%
- Body mass index (BMI): 17.9 to 53.0
- Reference measurements: Roche Contour Capillary blood samples, using always two separate test strips and monitoring devices for each data point.