News

Newly published research further validates GlucoModicum’s path to high-precision, non-invasive glucose monitor

Mpac Lambert Partners with GlucoModicum to Deliver Scalable Automation for Needle-Free Glucose Monitoring Sensor

FIMEA Authorizes GlucoModicum to Conduct Pre-Pivotal Clinical Investigation Under EU MDR Article 62

Eurofins Electric & Electronics Finland audits GlucoModicum-Sofio operations, with zero deviations.
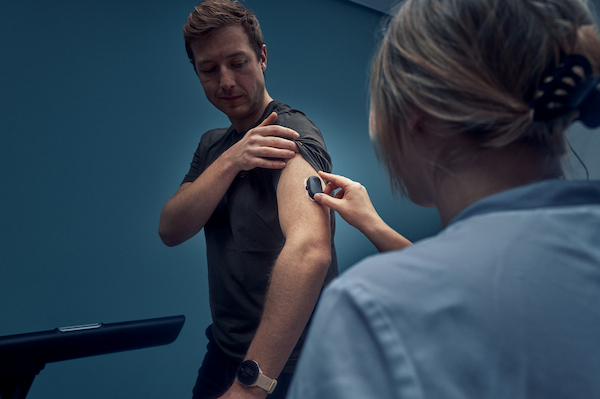
GlucoModicum Completes Clinical Performance Study of 646 Subject Visits and Reports Positive Results for Its Sofio Needle-Free Glucose Monitor

Major Medical Device Development Program Conducted with Phillips-Medisize Across the US and Europe to Advance Sofio

Newly published research further validates GlucoModicum’s path to high-precision, non-invasive glucose monitor

Mpac Lambert Partners with GlucoModicum to Deliver Scalable Automation for Needle-Free Glucose Monitoring Sensor

FIMEA Authorizes GlucoModicum to Conduct Pre-Pivotal Clinical Investigation Under EU MDR Article 62

Eurofins Electric & Electronics Finland audits GlucoModicum-Sofio operations, with zero deviations.

GlucoModicum Completes Clinical Performance Study of 646 Subject Visits and Reports Positive Results for Its Sofio Needle-Free Glucose Monitor

Major Medical Device Development Program Conducted with Phillips-Medisize Across the US and Europe to Advance Sofio